Polymer Clay works
by James Lehman
|
Painting In Space
|
The word polymer does not refer to a specific substance, but rather a phenomena of molecular structure that is observed in nearly an infinite number of different substances. POLY is of Greek origin and means many. MER means part or parts. Polymer molecules are made of many parts. "Carbon-based-life-form" isn't just a science fiction phrase!
Carbon is a very special element.


Other atoms may be connected to these four points. 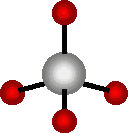
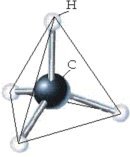
This compound of one carbon atom and four hydrogen atoms is methane gas (CH4). Carbon atoms bond to each other very well! 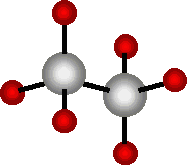
This compound of two carbon atoms and six hydrogen atoms is ethane gas (C2H6). It is also a simple example of a molecule made of parts. It is two methane molecules stuck together (sort of). 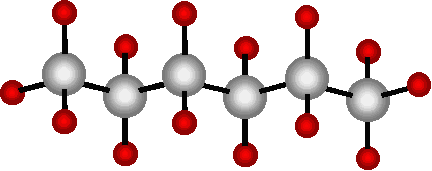
This is hexane (C6H14). It has six carbon atoms. Any number of carbon atoms can be found stuck together this way. The first ten hydrocarbons, molecules containing only carbon and hydrogen, are listed below. 1 Methane CH4 CH4 2 Ethane C2H6 CH3CH3 3 Propane C3H8 CH3CH2CH3 4 Butane C4H10 CH3CH2CH2CH3 5 Pentane C5H12 CH3CH2CH2CH2CH3 6 Hexane C6H14 CH3CH2CH2CH2CH2CH3 7 Heptane C7H16 CH3CH2CH2CH2CH2CH2CH3 8 Octane C8H18 CH3CH2CH2CH2CH2CH2CH2CH3 9 Nonane C9H20 CH3CH2CH2CH2CH2CH2CH2CH2CH3 10 Decane C10H22 CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3All of these compounds and many others are collectively called petrochemicals. They are all found in crude oil! The first four are gasses at normal atmospheric pressure, the next bunch are volatile liquids. They are all very flammable and commonly used as fuel. When seventeen or more carbons are found together in a hydrocarbon chain it is a solid material called paraffin wax. It is important to note that crude oil is organic waste. All of the crude oil and coal that exists today was once the life that inhabited the Earth millions of years ago. It has no food value to any living thing, but it is still 100% organic material. All of the atomic bonds between all the atoms within every molecule were assembled within living organisms. Over millions of years, these molecules were broken down by other organisms, heat, presure and radiation. When oil and coal burn, they release that last bit of energy that makes them organic. They are reduced to mainly carbon dioxide, water and a lot of heat. There is only one way carbon returns to organic molecules.
Animals and protozoa eat the plants and each other converting the organic materials they eat into new and different complex organic molecules. Polymers are everywhere!
Glucose molecules can link together end-to-end to form long chain polymers. One variation of this formation is called cellulose. Cellulose is the primary structural molecule of all plant life and therefore the single most common, by mass, organic polymer molecule on Earth. A puff of natural cotton is an example of nearly pure cellulose. Wood is no less than 70% cellulose. Rayon, Cellophane and Celluloid are examples of synthetic plastics that are actually cellulose polymer fibers that have been slightly altered by human polymer science and engineering. The shape of things
Polymers are alive!
As a matter of fact, if it was possible to remove all of the water and basic minerals from any living organism, all that would be left would be organic, polymer molecules. Vitalism vs. Mechanism
There are two opposing philosophies about this very subject: Mechanism is the idea that all chemistry, even the most complex DNA structures, can be explained in terms of ordinary mechanics and physics, some of which we just haven't discovered yet. Vitalism is the idea that in order to form even the simplest organic molecule, an immeasurable and unexplainable vital force must be present. 
Michelangelo, Creation of Adam So far, the debate continues.
So what does all of this have to do with polymer clay? When we apply human technology,
Now imagine that these polymer chains are millions of units in length. Not only are these molecules huge and heavy, but they are also long, kinky threads that wrap and tangle with each other. If we introduce an oily substance to these tangled threads, called a plastisizer, or diluent, the threads can slip and slide against each other much more freely. A pot full of cooked, wet spaghetti is a pretty good analogy. The noodles are the polymer threads and the water is the plastisizer. This mixture is almost liquid. This is the state of soft, uncured polymer clay. Unlike spaghetti, applying lots of force to the threads is unlikely to break them. At or near room temperature, PVC molecules are incredibly strong. Forcing them to move against each other causes them to elongate, spread out over a larger area and involve a greater number of threads in a tangle. Hand worked, well conditioned polymer clay is stronger and smoother. Just the slightest amount of heat gets the molecules really vibrating, making their movement around each other even easier. Hand warmed polymer clay gets softer. Even more heat will cause the plastisizer to evaporate. The threads will start to stick to each other and the whole substance will harden and fuse into tightly wrapped threads of PVC, hard plastic. Polymer clay is not alive
|